Why is Understanding pH important?
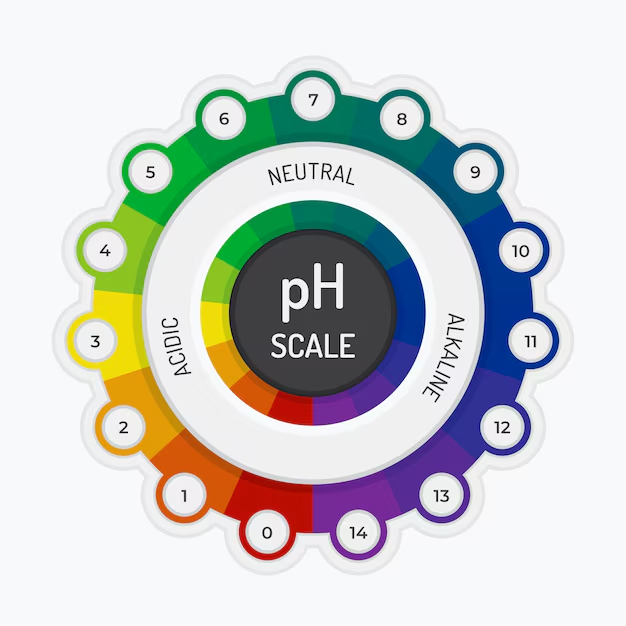
pH is a scale used to measure how acidic or alkaline (basic) a substance is. It stands for “potential of Hydrogen”. The pH scale ranges from 0 to 14, with 7 being neutral. A pH value below 7 indicates acidity, while a value above 7 indicates alkalinity. Understanding pH can be useful in daily life.
How Does the pH Scale Work by Understanding pH!
The pH scale is logarithmic, meaning each whole number represents a tenfold difference in hydrogen ion concentration. For example, a substance with a pH of 3 is 10 times more acidic than one with a pH of 4. The scale is based on the concentration of hydrogen ions (H⁺) in a solution by Understanding pH:
- Acidic substances (pH < 7) have a higher concentration of H⁺ ions.
- Alkaline substances (pH > 7) have a lower concentration of H⁺ ions and more hydroxide ions (OH⁻).
- Neutral substances (pH = 7), like pure water, have an equal balance of H⁺ and OH⁻ ions.
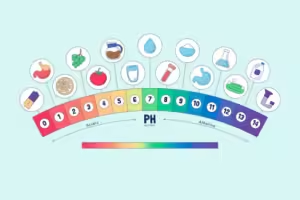
Examples of pH Levels in Everyday Life:
- Acidic: Lemon juice (pH ~2), Vinegar (pH ~3), Black coffee (pH ~5)
- Neutral: Pure water (pH 7)
- Alkaline: Baking soda (pH ~9), Soapy water (pH ~12), Bleach (pH ~13)
Why is pH Important?
- Biological Systems: Most living organisms, including humans, depend on maintaining a stable pH to function. Human blood, for instance, must stay around pH 7.4 for proper cellular functions.
- Agriculture: Soil pH affects the availability of nutrients to plants, impacting growth and crop yield.
- Environmental Monitoring: pH levels in oceans and rivers are crucial for the health of aquatic ecosystems. Ocean acidification, for example, threatens marine life as excess CO₂ lowers the pH of seawater.
How to Measure pH:
pH can be measured using:
- Litmus paper: Simple strips that change color based on the acidity or alkalinity of a solution.
- pH meters: More accurate digital devices that measure the hydrogen ion concentration in liquids.
Conclusion
Understanding pH is essential in various fields, from science and medicine to agriculture and environmental conservation. Whether testing pool water or analyzing soil health, pH helps us measure the balance between acidity and alkalinity, making it a fundamental concept in everyday life.
NOTE: – IT IS ALWAYS RECOMMENDED TO CONSULT YOUR HEALTH CARE PROFESSIONAL. ALL DATA SHARED HERE ARE FOR EDUCATIONAL PURPOSES ONLY.